Overview
Zinc-air batteries are a type of metal-air battery technology that offers the potential for high energy density, inherent safety, and the use of affordable, earth-abundant materials. These characteristics have attracted interest for a wide range of energy storage applications, from hearing aids and sensors to large-scale stationary energy storage. Most zinc-air batteries commercially available today are primary cells (single-use), but there is growing research and development effort aimed at making zinc-air batteries secondary cells (rechargeable). This work is driven by the technology's promise to combine high performance with sustainability and low cost.
Working Principle
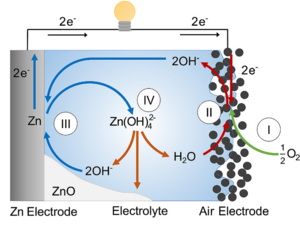
A typical zinc-air battery couples an oxygen reduction cathode with a zinc-based anode in an alkaline electrolyte. During discharge, oxygen from the air undergoes reduction at the cathode, while zinc metal at the anode is oxidized. In primary zinc-air batteries, the cathode is a porous gas diffusion electrode containing an oxygen reduction catalyst, such as manganese dioxide (MnO₂). The oxygen reduction reaction (ORR) proceeds according to:
O₂ + H₂O + 4e⁻ ⇌ 4OH⁻
Rechargeable (secondary) zinc-air batteries use a bi-functional air cathode capable of both the oxygen reduction reaction (ORR) during discharge and the oxygen evolution reaction (OER) during charging.
A schematic showing the working principle of an alkaline zinc-air battery cell. Reused with permission from N. Borchers, S. Clark, B. Horstmann, K. Jayasayee, M. Juel, and P. Stevens, “Innovative Zinc-Based Batteries,” J. Power Sources, 484, no. December 2020, p. 229309, 2021, https://doi.org/10.1016/j.jpowsour.2020.229309.